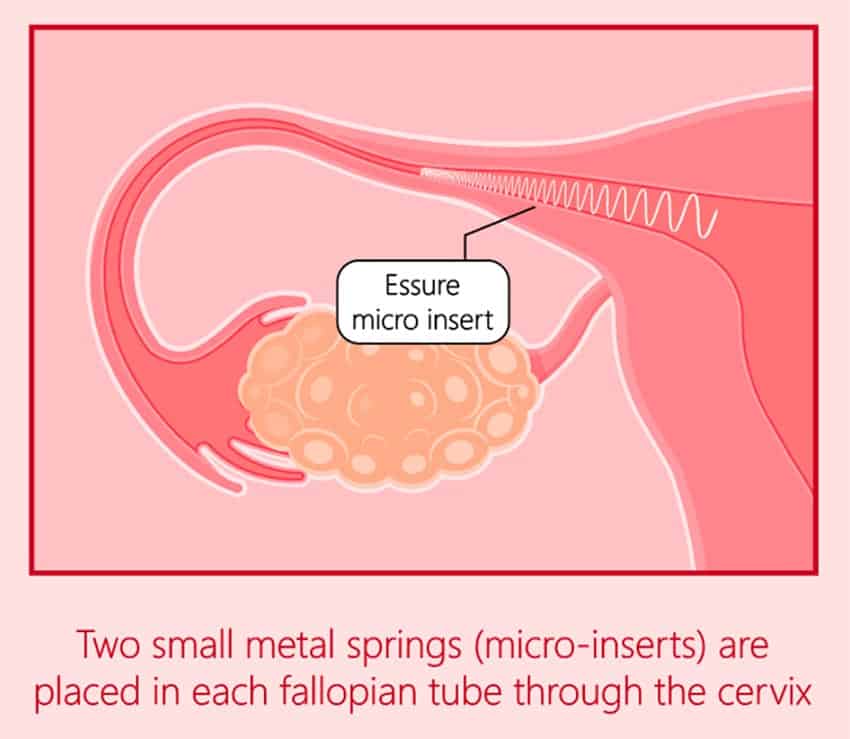
Essure Injuries & Problems
Since introduced to the market in 2002, over 26,000 complaints about Essure have been reported to the FDA. Injuries reported to the FDA include, but are certainly not limited to:
- Hysterectomy
- Unintended/Ectopic Pregnancy
- Breakage of One or Both Micro-Inserts
- Migration of one or both micro-inserts
- Nickel Poisoning (hair loss, rashes, metal taste in mouth, joint/muscle pain, tooth decay, etc.)
- Perforation of the Uterus, Fallopian Tube(s), and/or Other Pelvic/Abdominal Organs
- Abnormal/Heavy Menstrual Bleeding
- Severe Abdominal/Pelvic Pain
- Autoimmune Disorders
- Severe Menstrual Pain